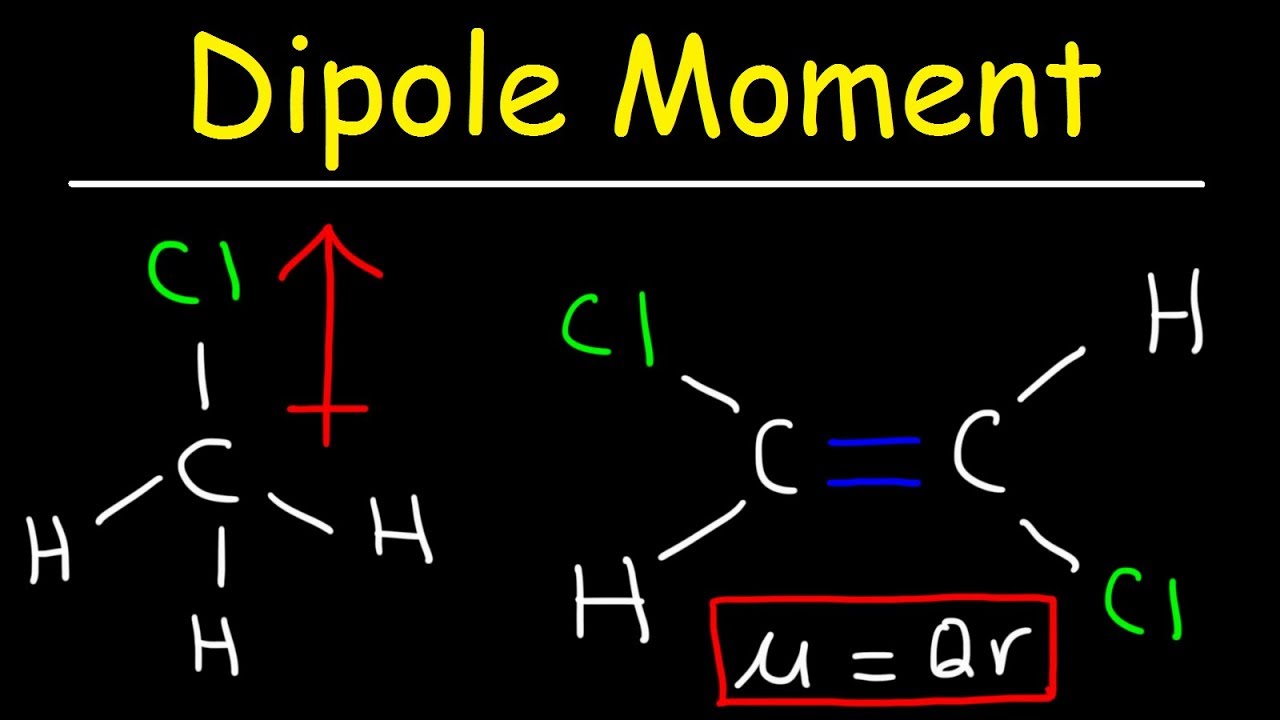
What Causes a Molecule to Have a Net Dipole Moment
CO₂ SO₂ CH₄ or BF₃. CHF_3 is a polar molecule.

How To Determine Whether A Molecule Has An Overall Molecular Dipole Moment Youtube
The separation of charges in any system leads to a dipole moment.
. The polarity that a molecule is directly proportional come the distinction in the electronegativity the atoms. The overall dipole moment of a molecule is obtained by vectorial addition of different bond moments which arise due to unsymmetrical distribution of bond pair electrons as shown below. Learn this topic by watching Molecular Polarity Concept Videos All Chemistry Practice Problems Molecular Polarity Practice Problems Q.
Is no net movement of electrons and a non-polar molecule with no net dipole moment. The total number of electrons around the central atom S is eight which gives four electron pairs. What is the hybridization of the carbon atoms in these compoundsb.
16 Suppose that a molecule has the formula AB 3. To find out which molecule has zero dipole moment we need the net dipole moment of the given compounds and for that we need to check the polarity of the compounds. Polar or nonpolar Q.
This occurs due to an atoms electronegativity - where one atom has the ability to attract electrons towards it In other words electrons wants to spend other time around it giving it a negative charge and the other a positive charge. A bond dipole moment is a measure of the polarity of a chemical bond between two atoms in a molecule. Only cis3-hexane is unsymmetrical molecule.
Dec 20 2017. A polar molecule has a net dipole as a result of the opposing charges ie. In CH 2 F 2 the hydrogen is less electronegative than the carbon this means there will be a net movement of electrons towards the fluorine and the molecule will be polar and have a net dipole moment.
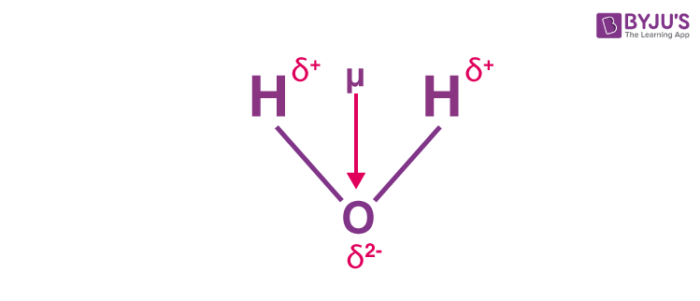
In an electric field polar molecules align with the _____ toward the. They can therefore arise in ionic bonds as well as in covalent bonds. In H_2O molecule the oxygen atom being much more electronegative than the hydrogen atom will cause the.
Has a perfect tetrahedral geometry with all side atoms same. The presence of both covalent and ionic bonds in the molecule. Draw the Lewis structures of CF4 and CF2CCl2a.
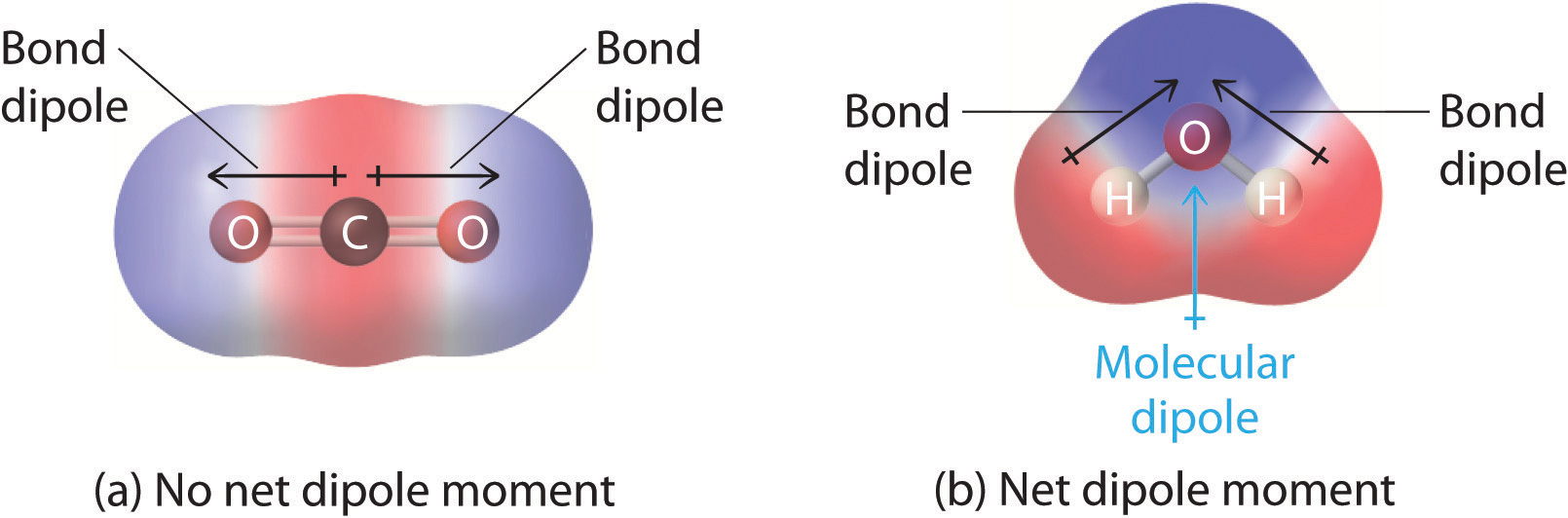
A dipole moment arises in any system in which there is a separation of charge. The bond dipoles cannot cancel one another so the molecule has a net dipole moment. A bond dipole moment.
See full answer below. This means that salicylic acid contains a benzene ring a hydroxyl group attached to one of the carbon atoms and a carboxylic acid group attached to. Does the molecule BF 2 Cl have a dipole moment.
Which molecule will have a net dipole. The main cause for the development of the dipole moment is the electronegativity difference between chemically bonded atoms or elements. As a molecule vibrates if there is a fluctuation in its dipole moment then this induces an electric field that interacts with the electric field associated with the infra red radiation.
Water H2O is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. Compound I and III have same g view the full answer. The presence of a single polar bond causes the molecule to have a net dipole moment μ μ because the electron density.
The presence of only nonpolar bonds in the molecule B. In O2 theres no separation of charges. The presence of Van der Waals forces C.
Both ionic and covalently bonded compounds develop dipole moments. The dipole moment of a molecule is the measure up of its polarity. 2 2 3 3 -Tetrammethyl butane.
Electronegativity of B A. What is the relationship between dipole moments and the magnitude of the partial charges. Having partial positive and partial negative charges from polar bonds arranged asymmetrically.
Generally Polar compounds do not have zero dipole moment. Two of these electron pairs are bonding pairs and two are lone pairs so the molecular geometry of H 2 S is bent Figure 226. Dipole moments occur when there is a separation of charge.
Dipole moments occur when there is a separation of charge. The compound which has same groups on both the carbons of CC will have less dipole moment than the compounds having different groups. In order for a molecule to have a net dipole moment it has to have an asymmetric separation of charges.
The polarity of a molecule is straight proportional to. Click to see full answer. Has a perfect tetrahedral geometry but due to the presence of dissimilar groups its net dipole moment is not zero.
Solve any question of Chemical Bonding and Molecular Structure with-. Ay molecule with a net dipole moment will have dipole -dipole interactions. If there is a match in frequency of the radiation and the natural vibration of the molecule absorption occurs.
Correct option is B Dipole moment is vector quantity. Only unsymmetrical molecules have dipole moment. Dipole moments occur due to atoms electronegativity where one atom has the ability to attract electrons towards it giving the electrons a negative and a positive charge.
The separation of charges in polar covalent bonds gives rise to. What causes a molecule to have a net dipole moment. Salicylic acid is also known as 2-hydroxybenzoic acid and 2-carboxyphenol.
A ll molecules will have London dispersion forces which get stronger as the molecule gets heavier more electrons causes a shift in electron cloud distribution resulting in a temporary dipole. In a molecule if there is a difference between the electronegativity of two atoms then there exist a polarity in the bond. The presence of a net charge that does not cancel out D.
Its asymmetrically placed carboxylic acid group and hydroxyl group cause it to have a net dipole moment which grants it its polarity. These are molecules with polar bonds caused by a diference in. Due to difference in electronegativities of the 2 atoms.
Dipole moments occur due to the difference in electronegativity between two chemically bonded atoms. The atoms are both neutral so the atom has no dipole moment. Due to symmetry this molecule has a net zero dipole moment.
It has dipole moment. So it will have dipole dipole interaction along with the weaker dispersion forces. HCl is a diatomic molecule with dissimilar atoms.

How To Determine Whether A Molecule Has An Overall Molecular Dipole Moment Youtube

The Net Dipole Moment Of A Polyatomic Molecule Depends On The Spatial Arrangement Of Various Bonds In The Molecule The Dipole Moment Of Bf3 Is Zero While That Of Nf3 Is Not

What Is Dipole Moment Definition Formula Facts Uses Examples

Why The Dipole Moment Of Ch3f Is Less Than Ch3cl Although F Is More Electronegative Than Cl Chemsolve Ne In This Moment Bond Length Chemistry Classroom

What Does A Net Dipole Mean Quora

Science Coverage Is Hcl Polar Or Nonpolar Covalent Bonding Molecular Geometry Molecules

Dipole Moment Molecular Polarity Percent Ionic Character Youtube
Dipole Moment Definition Detailed Explanation And Formula

Dipole Moment Definition Detailed Explanation And Formula

Water Molecular Dipole By Vector Sum Chemistry Lessons Chemistry Molecular

If Bcl Bond Has A Dipole Moment Explain Why Bcl3 Molecule Has Zero Dipole Moment
.png?revision=1)
2 2 Polar Covalent Bonds Dipole Moments Chemistry Libretexts

What Causes A Molecule To Have A Net Dipole Moment Quora
2 2 Polar Covalent Bonds Dipole Moments Chemistry Libretexts

Dipole Moments Mcc Organic Chemistry
Why Is A Molecular Dipole Moment Zero In Symmetrical Molecules Quora

I Discuss The Significance Applications Of Dipole Moment Ii Represent Diagrammatically The Bond Moments And The Resultant Dipole Moment In Co2 Nf3 And Chcl3

